Here’s an article by Ketan Desai of Internation Medical Consultants on Bioterrrorism stocks. Ketan likes Siga, which is very volitile, see chart below. MSRA is likely going to be a huge problem within 20 years, making biotechs involved with drugs for multi-drug resistant bacteria particulary interesting to follow as a group that could really run, in the right environment with the right news, (though if the news is bad enough, it won’t matter!). – Ilene
Bioterrorism: Smallpox and Biotech Companies
Since 2001, bioterrorism has become a well-recognized threat. The list of possible agents is long, and includes Bacillus anthracis (Anthrax), Clostridium botulinum (Botulism), Francisella tularensis (Tularemia), Variola Major (Smallpox), Yersinia Pestis (Plague), etc. Do companies that work on these threats represent an investment opportunity? I don’t plan to write on all these diseases – just two of the more visible ones. This article will concentrate on companies working on Smallpox and the follow up article will focus on Anthrax.
Smallpox
A virus, Variola Major, is the cause of Smallpox. The disease was declared eradicated worldwide in 1980; the last naturally occurring case was in Somalia in 1977. Routine vaccination against smallpox in the U.S. stopped in 1972, and Dryvax (made by Wyeth from Vaccinia, a relative of Variola) production was halted in 1982. After the eradication of smallpox, the World Health Organization (WHO) recommended that all remaining stocks of Variola virus be destroyed or sent to one of two designated reference laboratories: at the Centers for Disease Control and Prevention [CDC] in Atlanta, Georgia, or the Research Institute of Viral Preparations in Moscow, Soviet Union. It was discovered later that Russia moved its smallpox samples to VECTOR, a Siberian facility that had previously served as a biological weapons development plant.
 What makes Smallpox a deadly possible weapon is that it is relatively easy to disseminate, the virus is relatively stable as an aerosol (size less than 5 um), and the disease has a high mortality rate of about 30%. Aerosolized biological agents may remain suspended in air for long periods and may travel long distances. There are also many reasons why Variola is not a good weapon – I won’t go into them here, but if someone is really interested, they can read the reasons in my book Germs of War.
What makes Smallpox a deadly possible weapon is that it is relatively easy to disseminate, the virus is relatively stable as an aerosol (size less than 5 um), and the disease has a high mortality rate of about 30%. Aerosolized biological agents may remain suspended in air for long periods and may travel long distances. There are also many reasons why Variola is not a good weapon – I won’t go into them here, but if someone is really interested, they can read the reasons in my book Germs of War.
In order to encourage companies to develop drugs against Smallpox and other biological weapons, Congress passed the Public Health Security and Bioterrorism Preparedness and Response Act of 2002 (the Bioterrorism Act), which President Bush signed into law June 12, 2002. Thereafter, Project Bioshield Act of 2005 was passed into law. These two acts undertook measures to induce the pharmaceutical/biotechnology industry to come up with new drugs to combat bioterrorism and biological warfare. The industry had been reluctant to invest in this field – why spend millions (maybe billions) on something that may or may not ever be used? Who would be the buyer? And what about the legal aspects if something went wrong? Among other measures, these acts addressed the concerns of the industry to some extent.
Some companies, mainly biotechnology companies, took up the challenge and came up with vaccines and drugs to combat Smallpox. A few companies that I find notable are listed below.
Vaccines
Acambis makes ACAM2000™, a live vaccinia virus. Vaccinia is related to Variola, but does not cause Smallpox. Immunizing with Vaccinia results in immunity to Variola. This vaccine was developed under a contract with the US Centers for Disease Control and Prevention [CDC] and is made in cell culture (as opposed to eggs). It is the primary smallpox vaccine for use in a bioterrorism emergency and forms the majority of the US Government’s smallpox vaccine stockpile. Acambis has supplied 195.2 million doses of ACAM2000™ to the US Government for its Strategic National Stockpile. Acambis has also supplied ACAM2000™ under an FDA Investigational New Drug application to several other governments around the world. The Food and Drug Administration approved the vaccine in August 2007.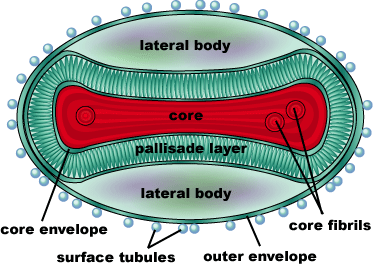
Acambis is also developing vaccines against Japanese Encephalitis (phase III completed, licensed to Sanofi-Pasteur), West Nile (phase II completed, licensed to Sanofi-Pasteur), and Dengue (phase III, licensed to Sanofi-Pasteur). It is the only company that is developing vaccines against C. Diff Colitis (caused by Clostridium difficile) and the product finished phase I in 2007. It is being reformulated and will go into phase II later in 2008. Perhaps the biggest of them all, it has a vaccine against Influenza that is not limited to a particular strain but attempts to elicit broad immunity. The company reported the results from its phase I study in January 2008.
Bavarian Nordic uses its modified vaccine Ankara (MVA-BN®) technology of multivalent vaccine vectors for the development of vaccines against infectious diseases. It has ongoing development contracts with the US government (awarded in June 2007, September 2004 and February 2003) to develop IMVAMUNE® as a safe third-generation smallpox vaccine. Bavarian Nordic’s clinical development program has been further expedited by the US government with the FDA’s grant of “fast-track” status for IMVAMUNE®.
To-date, the company has shown IMVAMUNE® to be the best characterized MVA based on:
- Comprehensive preclinical animal data (in 2 mice models and a non-human primate model)
- Completed phase I and II studies in healthy subjects, subjects with atopic dermatitis and HIV infected subjects
- Additional Phase II studies started in at risk populations (HIV, atopic dermatitis)
In addition to Smallpox, Bavarian Nordic is also developing a vaccine against Anthrax, (pre-clinical stage), HIV (phase I/II), and Measles (phase I/II). It has a vaccine program against breast cancer (phase I/II) and prostate cancer (phase I/II).
CJ Corporation in Republic of Korea has developed cell-culture derived Smallpox vaccine (CJ-50300) that is in phase III in South Korea.
Other companies that have had smallpox programs in the past include BioPort and Vaxgen.
Two licensed vaccines for smallpox are currently in the CDC Strategic National Stockpile [SNS] – ACAM2000 (Acambis) and “wetvax” (sanofi aventis (SNY)). The Sanofi-Aventis vaccine is an old vaccine that was somewhat mysteriously found in a freezer in 2002, and donated by the company to the government. As far as I know, it is not in production. The SNS contains more than 300 million doses of live vaccinia virus smallpox vaccine, or enough to immunize every person in the U.S. The HHS contract for the MVA vaccine will add 20 million doses, or enough to treat 10 million people with compromised immune systems (the vaccine requires a two-dose regimen per person).
About 95% of people are protected within 10 days of getting a single smallpox vaccination. Smallpox vaccination up to 3 days after someone is exposed to smallpox virus will prevent or reduce the severity of smallpox in most people. Vaccination 4 to 7 days after exposure will likely offer partial protection. Solid protection lasts for 3 to 5 years after first vaccination. Solid protection after revaccination lasts about 10 years. Partial protection lasts longer, but people need to be revaccinated if too much time has passed.
Drugs
Siga Technologies (SIGA) is developing ST-246, SIGA’s leading smallpox antiviral candidate. The compound works by blocking the transmission of the virus from cell to cell, and is not a vaccine. SIGA’s ST-246 has shown evidence of safety and high levels of efficacy against poxvirus disease in multiple animal trials, as well as continued success in human safety studies. On March 27, 2008, the company announced that it had finished its phase I studies and the drug was found to be safe. Daily dosing was feasible, and PK in blood was comparable to that in monkeys at which it was effective against smallpox. As a result of success in early trials and in one emergency compassionate use case, the FDA has designated ST-246 for "fast track" status.
Other compounds Siga is developing are in the pre-clinical stage and they include drugs for Ebola/Marburg, Lassa Fever and Junin. SIGA’s anti-bacterial program includes development of a new class of agents to combat varieties of Staphylococcus, Streptococcus, Enterococcus, and others that are highly resistant to existing antibiotics.
Of the three companies mentioned above, only Siga trades in the US. Its all time high, in 2001, was around $8/share. It trades around $2.88 at present. Acambis does not trade on Nasdaq or NY Stock Exchange. Its high at 370 GBp (Great Britain Pound) was in late 2003, and now trades around 114 GBp. Bavarian Nordic also does not trade in the US – its high was around 600 DKK (Danish Krone) in Jan 07 in the Copenhagen market. It now trades around 227.
It would be risky to buy any of these companies based solely on Smallpox. However, each of the three companies develops dugs/vaccines for other diseases. That is where I find them to be more attractive, especially Siga’s pipeline for drugs targeting resistant bacterial infections.  Keep in mind that on June 4, 2008, Novartis announced a deal to acquire Protez Pharmaceuticals for up to $400 million. The attraction seems to be Protez’s PZ 601, a compound that has a unique broad -spectrum of activity that could offer better coverage over existing injectable antibiotics, especially multi-drug resistant bacteria, including MRSA. Not bad for a company that was founded only in 2003.
Keep in mind that on June 4, 2008, Novartis announced a deal to acquire Protez Pharmaceuticals for up to $400 million. The attraction seems to be Protez’s PZ 601, a compound that has a unique broad -spectrum of activity that could offer better coverage over existing injectable antibiotics, especially multi-drug resistant bacteria, including MRSA. Not bad for a company that was founded only in 2003.


