Reminder: Pharmboy is available to chat with Members, comments are found below each post.
 Corporation (NASDAQ: CELG) is a manufacturer of drug therapies for cancer and inflammatory disorders. The Company was founded in 1986 from a spin-out of the Celanese Corporation's merger with American Hoechst Corporation.
Corporation (NASDAQ: CELG) is a manufacturer of drug therapies for cancer and inflammatory disorders. The Company was founded in 1986 from a spin-out of the Celanese Corporation's merger with American Hoechst Corporation.
Figure 1. History of Celgene
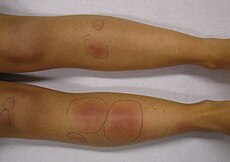
In 1998, the FDA approved Thalomid for the acute treatment of the cutaneous manifestations of moderate to severe erythema nodosum leprosum (ENL). In 2005, Revlimid was approved by the FDA for or the treatment of patients with transfusion-dependent anemia due to low- or intermediate-1-risk myelodysplastic syndromes (MDS) associated with a deletion 5q cytogenetic abnormality with or without additional cytogenetic abnormalities (mouthful I know!). The rest is history. Revlimid is their flagship product, and if all goes well, should eclipse $5B/yr in revenue by 2015.
Figure 2. ENL
Financials
Currently, at ~$57/share, the Company has a market cap of $26.6B and a trailing P/E of 30. In their most recent conference call, the stock has been range bound for several years, with highs in the mid-$70s and lows in the $40s, so the stock is a trend trader's dream. In their most recent conference call, the Company announced non-GAAP (Generally Accepted Accounting Principles) net income of $347.6 million, or non-GAAP diluted earnings per share of $0.73 for the quarter ended December 31, 2010. Non-GAAP net income for the fourth quarter of 2009 was $290.3 million or non-GAAP diluted earnings per share of $0.62. Based on U.S. GAAP, Celgene reported net income of $213.6 million, or diluted earnings per share of $0.45 for the quarter ended December 31, 2010. GAAP net income for the fourth quarter of 2009 was $254.2 million, or diluted earnings per share of $0.54.
"The 2010 record financial and operational results represent excellence in execution by all of our global teams," said Bob Hugin, Celgene's Chief Executive Officer. "This operating momentum, combined with our continued investment in R&D, positions us well for sustained growth in both the near and long term."
In mid-February 2011, the CELG announced that the Company's Board of Directors authorized the repurchase of up to an additional $1B common stock through December 2012. This authorization is in addition to the $0.5B authorization announced on January 10, 2011. The $0.5B program announced in April 2009 was fully utilized.
Noted in Table 1 below, the Company's major products and 2010 sales are:
- Thalomid (thalidomide), which is approved for the acute treatment of the cutaneous manifestations of moderate to severe ENL, as well as in combination with dexamethasone for patients with newly diagnosed multiple myeloma, and
- Revlmid (lenalidomide), for which the company has received FDA and EMEA approval in combination with dexamethasone for the treatment of multiple myeloma patients who have received at least one prior therapy. Revlimid is also approved in the US for the treatment of patients with transfusion-dependent anemia due to low- or intermediate-1-risk MDS associated with a deletion 5q cytogenetic abnormality with or without additional cytogenetic abnormalities.
- In addition, CELG receives royalties from NVS on sales of the entire Ritalin family of drugs (for ADHD).
Table 1. 2010 Sales of Celgene's Drugs and Projected Sales to 2015 (from 2011 10-K and forward looking statements).
Blue – not on the market yet.
In the beginning of March, CELG announced that the US Food and Drug Administration (FDA) will review the supplemental New Drug Application (sNDA) for cancer drug Istodax (romidepsin; Astellas owns the rights) on a priority basis. Istodax is a histone deacetylase (HDAC) inhibitor and is indicated as an iv infusion for the treatment of cutaneous T-cell lymphoma (CTCL) in patients who have received at least one prior systemic therapy.
CELG has four other compounds in Phase 3 clinical trials which could improved forward looking revenues. They are noted below:
- pomalidomide – under license from EntreMed, is developing pomalidomide, an orally available small-molecule analog of thalidomide that inhibits TNF-alpha overproduction, for the potential treatment of solid tumors including prostate cancer, multiple myeloma (MM), small-cell lung cancer (SCLC), and pancreas cancer. It is also being investigated for the potential treatment of graft versus host disease (GVHD), sickle cell anemia, myelofibrosis, polycythemia, and scleroderma. In several Phase 2 trials, the first in December 2008, pomalidomide combined with low dose dexamethasone in patients with relapsed or refractory MM, were presented at the 50th ASH meeting in San Francisco, CA. The combination of pomalidomide + dexamethasone well tolerated and working well, with an objective response rate of 62%, including a 29% response rate amongst patients with Revlmid-refractory MM. Similar data were reported at the 51st ASH meeting in New Orleans, LA in December 2009. The Phase 2 portion was ongoing and anticipated to complete in the fourth quarter of 2010. Further randomized and novel combination studies were also expected and in June 2010, data from the phase II trial were presented at the 46th ASCO meeting in Chicago, IL. The overall response rate was 54%, with 17% of patients achieving a very good partial response, 17% a partial response and 23%a minor response. At the 6-month follow-up, the progression-free survival rate, the overall survival rate and the median progression-free survival period were 58%, 86% and 8 months, respectively. Like, Revlmid and Thalomid, this drug should garner approval.
- apremilast – is an oral, small-molecule PDE4 inhibitor that modulates the production of proinflammatory mediators (including PDE 4, TNF alpha, IL-2, IL-6, IL-12, IL-17, IL-23, IFN gamma, and leukotrienes). CELG is investigating apremilast in three Phase 3 trials for psoriasis (includingpsorisis, psoriatic arthritis and recalcitrant psoriasis), Behcet's disease, and other chronic inflammatory conditions (e.g., lupus erythematosus, erosive osteoarthritis, uveitis, ankylosing spondylitis, prurigo nodularis, atopic dermatitis, allergic contact dermatitis and sarcoidosis (YES, that is a lot of diseases this could work for)). There is a ton of competition in this area, including GSK and MRK. It remains to be seen IF this compound class will work.
- amrubicin – Sumitomo Pharmaceuticals (now Dainippon Sumitomo) developed and launched (in Japan) amrubicin (doxorubicin prodrug named Calsed), which is an iv anthracycline topoisomerase II inhibitor for the treatment of small- and non-small-cell lung cancers (SCLC and NSCLC). CELG is developing the drug for the treatment of SCLC in the US and EU. It works, but how well compared to/with the standard of care?
- sotatercept – with Acceleron, CELG is developing this compound, which is a fusion protein containing a soluble form of the activin IIa receptor that inhibits signalling of the receptor and an Fc fragment of IgG1, for the subcutaneous treatment of chemotherapy-induced anemia, renal anemia and bone loss. In December 2009, Phase 2 trial data were presented at the 51st ASH meeting in New Orleans, LA. Patients received 0.1, 0.3 and 0.5 mg/kg sotatercept subcutaneous or placebo. After the first dose of sotatercept, there was a dose-dependent increases in hemoglobin. Sotatercept-treated patients demonstrated a greater trend to improvement in osteolytic lesions. Of 22 evaluable patients, complete remission or very good partial remission was seen in 7 patients.
Seven others to note that are in Phase 2, but are too early to count on. Many started trials over the last year or two, so data should be slowly trickling in, but larger, Phase 3 trials will be needed to move them forward. The drugs under development are:
- CC-223 – an mTOR inhibitor (think ARIA)
- Docetaxel – a nab-docetaxel, an iv nanoparticle albumin-bound (nab) formulation of the microtubule stabilizer docetaxel, for the potential treatment of solid tumors (think IMGN or Abraxane from above)
- CC-11006, a small molecule TNF-alpha synthesis inhibitor, an orally available immunomodulatory drug (related to thalidomide) for the treatment of hematological malignancies including myelodysplastic syndrome (nothing new)
- CC-930 (small molecule, oral delivery) is a lead from a series of inhibitors of cJun N-terminal kinase (JNK), for the treatment of serious inflammatory and fibrotic diseases (WAY to early, and competition galore).
- Cenplacel-L is one of many human placenta-derived stem cell (HPDSC) or human placental-derived adherant cell (HPDAC) therapies, for the potential iv treatment of malignant hematological diseases and other non-malignant disorders, including Crohn's disease, ulcerative colitis (UC), rheumatoid arthritis (RA), multiple slerosis (MS) and stroke.
- CC-11050 is an oral, small-molecule, tumor necrosis factor (TNF)-alpha and PDE4 inhibitor for the treatment of inflammatory disease including cutaneous lupus erythematosus.
- azacitidine is an oral, small molecule whose mechanism of action is as a hypomethylating agent and DNA methyltransferase inhibitor for the treatment of cancer.
Celgene has a very robust pipeline, and Revlmid is going to be the main player for them. IF any one of these other compounds show robust activity across several cancers, then CELG is going to be in a position of handsome returns. Much like our CEPH play, and knowing the range bound price of the stock, I like playing conservatively, buying the January 2012 $50/55 BCS for $3.35 or better, selling the January 2012 $5 Ps for $3. That is a $5 spread for $0.35. Earnings should keep the stock range bound, and any upside beat will make this one a nice winner in time.


